Medical Devices
Design, Development, and Approval
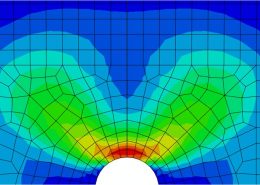
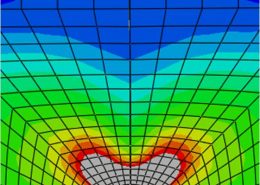
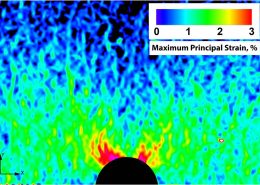
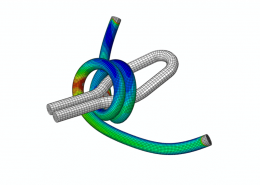
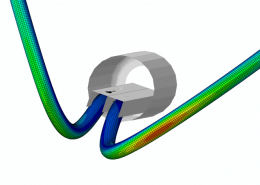
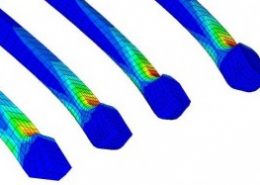
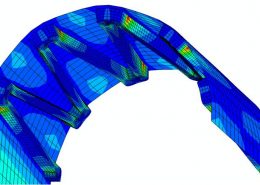
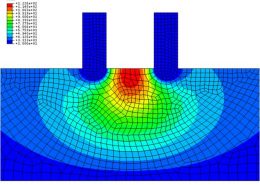
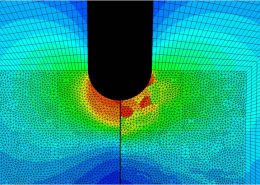
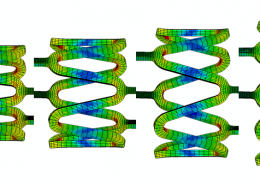
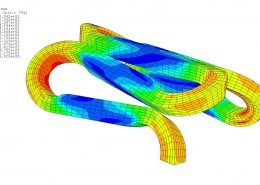
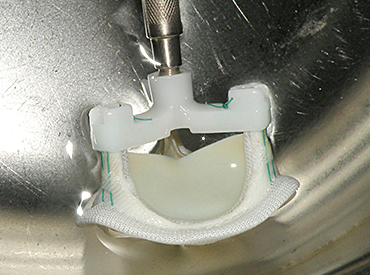
ECHOBIO LLC is experienced in all aspects of engineering implantable medical devices and provides a range of expert services. Our medical implant design and Finite Element Analysis (FEA) expertise is backed by over twenty years of hands-on mechanical testing and physical prototyping.
We specialize in validating medical device designs with FEA and getting regulatory approval for your product. From conception through PMA approval, we will help you minimize risk and meet development time-lines.
Find out how we can move you closer to getting regulatory approval.
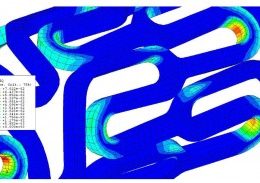
Let us identify opportunities for leveraging advanced engineering analysis to your application. Our experience includes fully coupled multi-physics, Finite Element Analysis (FEA), Computation Fluid Dynamics (CFD), Fluid Structure Interaction (FSI), RF, heat and mass transfer, modal analyses, optimization studies, etc.
We provide:
- FEA for Regulatory Submissions
- Design Optimization
- Product Sizing
- Boundary Condition Studies
- Manufacturing Studies
- Stress, Fatigue and Failure Analysis
Learn about custom and automated work flows for leveraging medical imaging analysis.
Ask about fatigue analysis , devices, design and optimization, test protocols and safety factor methodology. We offer customization including the development of user material subroutines (umats) including for example, NiTi, biological and other materials, polymers, blood, tissue, bone, etc.
Learn more about our engineering analysis services for medical devices.
- CE Mark
- HDE = Humanitarian Device Exemption
- 510k = Investigational Device Exemption
- PMA = Pre-Market Approval
Want to learn more?