Fatigue to Fracture at the FDA
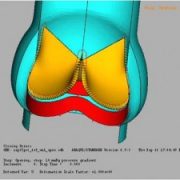
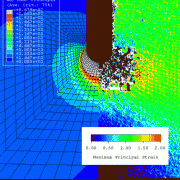
The gStent-BE is shown here, bending over backwards to make a point in a proposed ASTM standard that was discussed today at FDA headquarters. The draft guidance standard, passionately referred to as “Fatigue to Fracture”, is supported by engineers and regulators and has deep roots in the dialog between agency and industry. The standard addresses language and methodology related to durability testing, fractures and design validation for new devices.
The ASTM task group plans to send an edited version to the F04 committee for ballot early this summer. You are welcome to join ASTM and participate in this and other standards being revised and developed.